Dr. Matthias Kübel.
Image: Private archive.Dr. Matthias KÜBEL
Email: matthias.kuebel@uni-jena.de
Phone: +49 3641-9-47208
Dr. Kübel leads an Emmy-Noether research group Molecular Movies at the Institute of Optics and Quantum Electronics at the University of Jena. The central goal of the research group is to create Molecular Movies which visualize how molecular structure undergoes transformations, as for example during chemical reactions. This goal requires techniques that provide both ultrahigh spatial and temporal resolution. Moreover, since molecules consist of electrons and nuclei, it is desirable to track the motion of both types of particles simultaneously. We meet these challenges by combining state-of-the art femtosecond light sources with a coincidence spectrometer for detecting ionic fragments as well as photoelectrons.
Research Areas
Dr. Kübel’s research is rooted in strong-field physics, with an emphasis on coincidence spectroscopy. His interests include
- Time-resolved molecular imaging
- Manipulating electronic and chemical dynamics using phase-controlled laser fields
- Correlated ionization dynamics
- Few-cycle laser pulses
- Harmonic generation and interferometry
Teaching Fields
- Strong-field and attosecond physics
- Non-linear optics
Research Methods
- Cold-Target Recoil Ion Momentum Spectroscopy (COLTRIMS)
- Velocity map imaging
- Multi-color laser fields and femtosecond streaking
- Pump-probe techniques
Recent Research Results
We have recently recorded a movie of electron motion inside the valence shell of an argon ion. In our experiment [1], the ion is prepared in a quantum beat of two fine-structure states. This causes an electron hole to migrate from a peanut-shaped orbital () to a donut-shaped orbital ( and back in only 23 femtoseconds. Consequently, the electron density varies as a function of time, which is reflected in the experimental results presented in Movie 1. The data was recorded using three well-controlled laser pulses and a COLTRIMS apparatus to detect ions and photoelectrons in coincidence. A picture of our new apparatus is presented below.
Recorded snapshots of the relative electron density in the valence shell of an argon ion, modulated by a quantum beat of two fine structure states [1].
Illustration: Kübel research groupKey to this achievement was the usage of a previously developed streaking method [2] where a mid-infrared laser is used to deflect electrons created in its presence. This provides us with a versatile techniques which we want to employ in the future to create Molecular Movies. Interestingly, this new technique can be combined with the capability of COLTRIMS to measure ionic fragments and retrieve the nuclear structure from these measurements.
An example of a molecular movie that shows the motion of nuclei during a chemical reaction is presented in Movie 2. Here, a hydrogen migration takes place in an acetylene ion, such that acetylene (HCCH) isomerizes to vinylidene (CCH2). Our measurement [3] essentially measures the angle between the two CH bonds and how it varies with time. One can clearly see how the bond angle is initially close to 180° and then shrinks as the hydrogen atom is allowed more time to migrate before it is probed.
Images of the nuclear geometry of acetylene during isomerization to vinylidene, measured by time-resolved Coulomb explosion imaging [3].
Illustration: Kübel research group[1] M. Kübel, et al., Nat. Commun. 10, 1042 (2019).
[2] M. Kübel, et al., Phys. Rev. Lett. 119, 183201 (2017).
[3] C. Burger, et al., Faraday Discuss. 194, 495 (2016).
[4] M. Kübel, et al., Nat. Commun. 11, 2596 (2020).
[5] K. Amini, et al., , Eur. Phys. J. D 75, 275 (2021).
[6] M. Kübel, et al., Phys. Rev. Lett. 126, 113201 (2021).
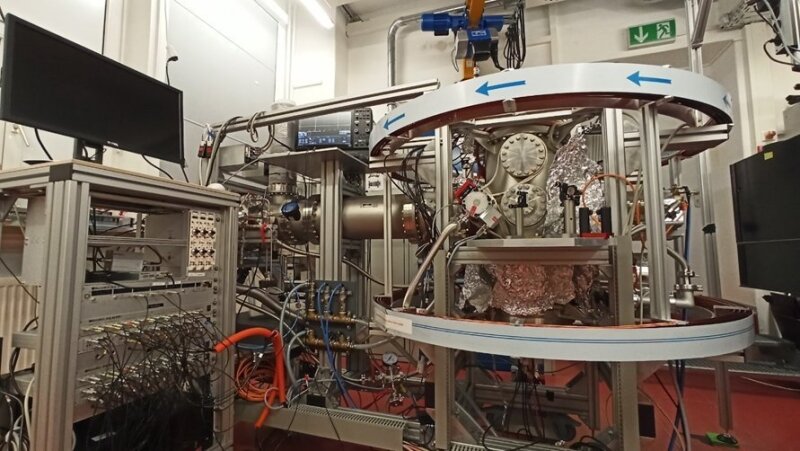
In 2020, we have set up a new COLTRIMS apparatus at the Institute of Optics and Quantum Electronics (IOQ). It will serve as the “camera” for our Molecular Movies. Here is a picture:
COLTRIMS apparatus at the Institute of Optics and Quantum Electronics (IOQ), which serves as the "camera" for our Molecular Movies.
Image: Jens Meyer (University of Jena)
![Recorded snapshots of the relative electron density in the valence shell of an argon ion, modulated by a quantum beat of two fine structure states [1].](https://www.acp.uni-jena.de/acpmedia/10142/kuebel-research-figure-movie1.jpg?height=137&width=500)
![Images of the nuclear geometry of acetylene during isomerization to vinylidene, measured by time-resolved Coulomb explosion imaging [3].](https://www.acp.uni-jena.de/acpmedia/10153/kuebel-research-figure-movie2.jpg?height=131&width=492)